Here is presented innovative solution of pvd deposition of corrosion resistant coatings onto black steel.
Structural steel, even when working in standard atmosphere is exposed of corrosion. The most common method of corrosion protection is an electrochemical galvanizing. However, this traditional technology has a number of drawbacks, the main of which are the ecological trouble and the low quality of the protective functions. We have developed an alternative technology of the corrosion-resistant coatings deposition onto structural steel, which does not use or require the subsequent disposal of harmful chemical compounds. The new technology is less expensive in comparison with electroplating. It requires several times smaller production areas when equal volumes of production, needs no treatment facilities.
Directly protective coating material the chromium-nickel stainless steel, is consumable in this technology. At the same corrosion resistance of a coating exceeds the relevant characteristics of coatings produced in electrochemical electroplating. The protective properties of coatings are preserved in the machining of steel sheets, including the hot forging, polishing, as well as resistance welding.
The developed technology is based on patented ion-plasma sources for processed products surface preparation and activation, so for the proper application of the coating film. Both sources are compatible in operating pressure, so they can work in parallel in interconnected vacuum process chambers. The principal features of this technology allow its wide implementation as follows. First, there is the possibility of obtaining the high quality corrosion-resistant coatings when working in an atmosphere of residual air in forevacuum pumping. Second, high adhesion and speed are provided by the deposition onto the grounded products without any additional heating of the substrate. Both of these factors greatly simplify and make cheaper the technical implementation of this technology
In proposed technology, finish cleaning and activation of the sample surface is carried out without its heating by the plasma flow through the processing of specialized helicon source with the flat antenna. Deposition of the protective film is made by the plasma arc source (International Application PCT/UA 2014 000011 of 27.01.2014, GreSem Innovation LLC). When processing by two sources the heterogeneity of film thickness surface is not less than ± 2.5% with a width of the treatment zone up to 1000 mm.
The patented helicon plasma source is a three-resonator system. Due to increase of efficiency of RF energy transfer to the plasma electrons, parameters of the ion-plasma flow which process the product surface, improved significantly.

Plasma-arc source of directed metal plasma flow works both by localizing the arc near the cathode working surface and subsequent plasma acceleration in electric and magnetic fields of the corresponding configuration. The source design includes the original non-invasive method of initiating of the arc discharge. This ensures the high reliability and process ability of the plasma arc source.
The plasma source specifications and parameters of the formed gas and metal plasma flows are presented in Table 1. The coating properties are presented in Table 2.
Adhesion, composition, structure and protective properties of the coatings deposited onto the substrates of structural steel Fe37-3FN with use of consumable stainless steel X10CrNiTi18-9 cathode were investigated.
Adhesion of the films was determined by repeatedly bending the substrate at the angle of 90º before fracture. The coating composition was investigated by the X-ray fluorescence spectrometer VRA-20 (Germany) and the TOF mass spectrometer with a laser ion source. The film structure was studied by the X-ray diffraction (XRD) by means of the ARL X’tra (Thermo scientific) equipment. Resistance to electrochemical corrosion of coatings was determined by the polarization measurements (chronopotentiometry, voltammetry).
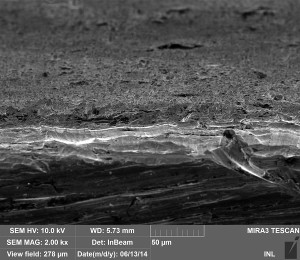
Results of the study are shown in the photomicrograph of adhesion substrate fracture shown in photomicrographs Figs. 2. Clearly, at nine-bending of the substrate after its break, there are no signs of delamination of the deposited film. And the film boundary reproduces accurately the fairly well developed surface topography of the substrate. Achieved adhesion allows polishing, bending and stamping of coated substrates without affecting its protective properties.
The results of analysis of the coating composition in comparison with the composition of consumable cathode by ray fluorescence method are given in Table 3.
Registered a slight increase of the chromium content in the coating, it may be due to increase of the evaporation of chromium in the arc micro spots and possibility of its sublimation. Excess of chromium in the coating in comparison with a consumable cathode is positive factor, since it determines the corrosion resistance of chromium stainless steel. Mass spectrometric measurements fully confirm this fact.
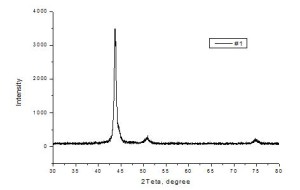
The X-ray analysis data shown in Fig. 3, demonstrated that unlike the cathode material, a peak at ~44.78º is present in radiographs of the tested coatings samples, that indicates the presence in the coating of the cubic iron with parameter a = 2.8608. In addition, depending on the coating deposition modes, it was fixed the cubic iron with larger dimensions of the cells, and appearance of hexagonal phase with a = 2.45 and c = 3.93. Obtaining of multiphase films further indicates that the coating film is formed in the non-equilibrium conditions, when substrate surface exposed by the intense accelerated ion flows of the gas and metal plasmas.
Coatings protective properties were investigated on the samples of structural steel Fe37-3FN with the following chemical composition in %: C – 0,14-0,12; Mn – 0,40-0,65; Si – 0,12-0,30. The protective coating deposited from the consumable cathode made of chrome-nickel stainless steel X10CrNiTi18-9 with the composition given in Table 3.
Initial information about the protective properties of corrosion-resistant coating can be obtained by measuring current-free potential in a corrosive environment In studies of samples obtained with new technology, the potentials are measured in a standard solution of 3 % sodium chloride, with respect to saturated silver chloride reference electrode, with recalculation to hydrogen scale. Fig. 4 shows the corresponding results for the stainless steel, from which the consumable cathode is made (curve 1), for the coated steel sample (curve 2) and steel to be coated (curve 3).
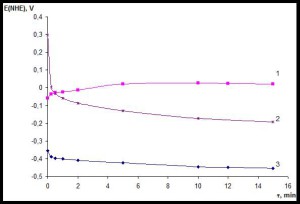
As follows from Fig. 4, the potential of the substrate material displaces over time in negative direction, indicating its active state in the corrosion solution. The stationary potential value is set at -0.45 V, which is typical for iron in neutral solutions. Stainless steel corrosion potential has a much more positive value, due to its oxidation and passivation.
Initial potential of steel with protective coating is even more positive than that of stainless steel. This indicates that the coating material is passive and more oxidized than the bulk material of the consumable cathode. However, over time, the potential of corrosion of steel coated shifted in the negative direction, as the potential of the substrate material. This suggests the gradual penetration of the solution towards the surface of the substrate material, i.e. the existence of micropores in the coating. The stationary value of the corrosion potential of the metal coated is intermediate between the potentials of the substrate material and the material of the consumable cathode.
From these data we can conclude only about that there are the pores in the coating, but not about the corrosion rate. Estimation of the rate of dissolution of the substrate metal is expedient to make after chrono-volt-amperometric studies. Fig. 5 shows the anodic polarization curves obtained in 3.5% solution of NaCl.
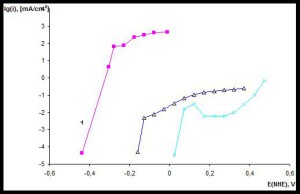
Anodic polarization curve for stainless steel (curve 3 in Fig. 5) has the form inherent in metals that are prone to passivation. Corresponding dependence of the sample coated with a protective coating (curve 2 in Fig. 5) is similar to the dependence of the corrosion current capacity for stainless steel. And this protective coating applied as a stainless steel coating, reduces the corrosion rate almost two orders of magnitude. A slight difference in the behavior of curves 2 and 3 in Fig. 5 is consistent with the presence of low porosity of the coating. In a first approximation, the porosity can be estimated from the ratio of the corrosion currents of the coated material and the substrate, which gives a value of about 0.02%. This amount of porosity by almost two orders of magnitude lower than that typical for the electroplating of zinc, as well as other not galvanic methods for corrosion protective coatings production. Stainless steel protective coating with a thickness over 10 microns deposited by the proposed technology, in its behavior is close to monolithic stainless steel, and this provides nearly 100% corrosion protection of the substrate surface. The coating protective properties are the same under conditions of electrochemical corrosion and in chemically active environments, such as seawater.
Stainless steel protective coatings produced by the proposed ion-plasma technology can be effectively applied in touring car automobile production, shipbuilding, while protecting pipelines operating at elevated electrochemical corrosion with “stray” currents in areas saturated with electricity cables.
The company GreSem Innovation, LLC expresses appreciation to the staff of the laboratories were involved into investigations of composition, structure and corrosion protective properties of the stainless steel coatings. The Heads of these laboratories are: Oleg Kolesnik from the Taras Shevchenko Kiev National University, Professor Vasilii Kladko from the Institute for Semiconductor Physics of the National Academy of Sciences of Ukraine, Professor Olga Linyucheva from the National Technological University of Ukraine of Kiev Polytechnic Institute.





Possibly layering other alloys of stainless steels with certain other base layer alloys allows much thinner effective coatings well under 1 MIL .
Many tight tolerance machine components must fit together at under 1/10-5 inch tolerance and seal at much tighter distances between surfaces when functioning in their designed environment such as reciprocating pistons , valve seats , pump seals , etc.
This means the coatings must be very thin , provide excellent corrosion resistance and have high lubricity at high pressure and temperature levels which is exactly what certain coatings for CRA materials do . If the CRAsteel itself and those coatings can be combined to coat mild steels at thinner thicknesses then this would be highly effective for corrosion resistance in many applications .
We have done exactly that .